Professor Shou-Hang Bo of the University of Michigan-Shanghai Jiao Tong University Joint Institute (UM-SJTU JI) and his collaborators have recently published a paper entitled “High magnesium mobility in ternary spinel chalcogenides” in Nature Communications, describing the discovery of the fastest magnesium-ion solid-state conductor (magnesium scandium selenide), a major step towards making solid-state Mg batteries a reality. The lead authors of this paper are Dr. Pieremanuele Canepa of Lawrence Berkeley National Laboratory and Professor Shou-Hang Bo. The other corresponding author is Professor. Gerbrand Ceder, Member of National Academy of Engineering, U. S., Senior Faculty Scientist of Lawrence Berkeley National Laboratory, Chancellor’s Professor of University of California, Berkeley.
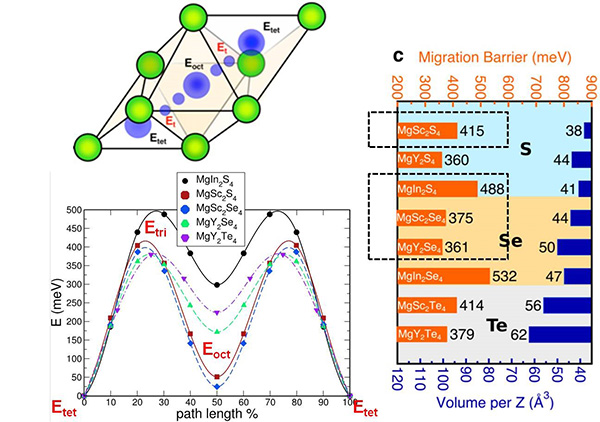 Computed magnesium ion migration barriers for a class of promising Mg conductors
Computed magnesium ion migration barriers for a class of promising Mg conductors
Mg batteries are both energy dense and safe and could compete with the current commercial Li-ion devices. The material described in Bo’s paper has the magnesium mobility comparable to solid-state electrolytes for lithium batteries. In all commercial batteries, a liquid electrolyte, which carries charges between the positive and negative electrodes, is used. The speed at which the ions (e.g., lithium or magnesium ions) are transported determines the rate to charge the battery. While permitting excellent ion transport, liquid electrolytes are flammable. A solid-state electrolyte would be far more fire-resistant; however, it faces the challenge to achieve fast ion transport.
Magnesium ion batteries offer even higher volumetric energy density than their lithium equivalents. However, finding a proper liquid electrolyte is difficult, because most electrolytes are either corrosive to current collectors or reactive to the magnesium metal negative electrode or cathode materials to form passivating interfaces, thereby shutting down the magnesium transport completely. Magnesium batteries are such a new technology that no good liquid electrolytes have been identified to date which can realize the promised high energy density. Bo’s research team tackled this obstacle from a different angle, i.e., making a solid-state magnesium conductor to alleviate the complex problems associated with liquid electrolytes.
Bo remarked, “making a solid-state electrolyte for magnesium batteries was a wild plan at the first sight.” “There probably is a long way to go before one can make a battery out of it, but we demonstrated for the first time that one can make solid-state materials with really good magnesium mobility through it,” commented Ceder. “Magnesium is thought to move slowly in most solids, so nobody would think it is possible.” Canepa added, “with a concerted effort bringing together computational materials science methodologies, synthesis, and a variety of characterization techniques, we have identified a new class of solid conductors that can transport magnesium ions at an unprecedented speed.”
Despite the exciting prospects, Ceder cautioned, “there are enormous efforts in industry to make a solid-state battery. It’s the holy grail because you would have the ultimate safe battery, but we still have work to do. This material shows a small amount of electron leakage, which has to be removed before it can be used in a battery.”
Other co-authors of the paper are Baris Key from Argonne National Laboratory, Gopalakrishnan Sai Gautam and Juchaun Li of Berkeley Lab, William Richards and Yan Wang of MIT, and Tan Shi and Yaosen Tian of UC Berkeley.
This work was fully supported as part of the Joint Center for Energy Storage Research (JCESR), an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Science, and Basic Energy Sciences. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Background information
 Dr. Shou-Hang Bo received his B. S. degree in Chemistry from Fudan University and a doctoral degree in Chemistry from Stony Brook University, under the supervision of Professors Clare Grey and Peter Khalifah. Since 2014, Bo had been a postdoctoral fellow in Professor Gerbrand Ceder’s group at the Department of Materials Science and Engineering, Massachusetts Institute of Technology, and the Materials Sciences Division, Lawrence Berkeley National Laboratory. Bo joined the Joint Institute in July 2017 as a tenure-track assistant professor. His recent research interests include (1) material- and system-level studies of solid-state materials for energy storage devices, and (2) real-time spectroscopic and scattering studies of inorganic material synthesis.
Dr. Shou-Hang Bo received his B. S. degree in Chemistry from Fudan University and a doctoral degree in Chemistry from Stony Brook University, under the supervision of Professors Clare Grey and Peter Khalifah. Since 2014, Bo had been a postdoctoral fellow in Professor Gerbrand Ceder’s group at the Department of Materials Science and Engineering, Massachusetts Institute of Technology, and the Materials Sciences Division, Lawrence Berkeley National Laboratory. Bo joined the Joint Institute in July 2017 as a tenure-track assistant professor. His recent research interests include (1) material- and system-level studies of solid-state materials for energy storage devices, and (2) real-time spectroscopic and scattering studies of inorganic material synthesis.





